
Lewis Structure Practice Worksheet 2 Answers Worksheet QA
This online quiz is intended to give you extra practice in identifying and drawing Lewis dot structures as well as predicting ion formation This quiz aligns with the following NGSS standard (s): HS-PS1-1 Select your preferences below and click 'Start' to give it a try!
Lewis Structures Worksheet With Answers
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.

Drawing Lewis Structures Worksheet in 2021 Worksheets, Geometry worksheets, Educational worksheets
When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share electrons until they are surrounded by eight valence electrons (an octet). Created by Sal Khan. Questions Tips & Thanks Want to join the conversation? Sort by: Top Voted Peter Patterson 3 years ago

Lewis Structure Practice Worksheet
Practice Problems Answer the following questions and check your answers below. These problems are for practice only will not be graded. Be sure you know how to draw correct Lewis Dot Structures and are able to correctly predict the electronic arrangement and molecular geometry before going on to the lab assignment.
Lewis Structure Worksheet With Answers Handmadefed
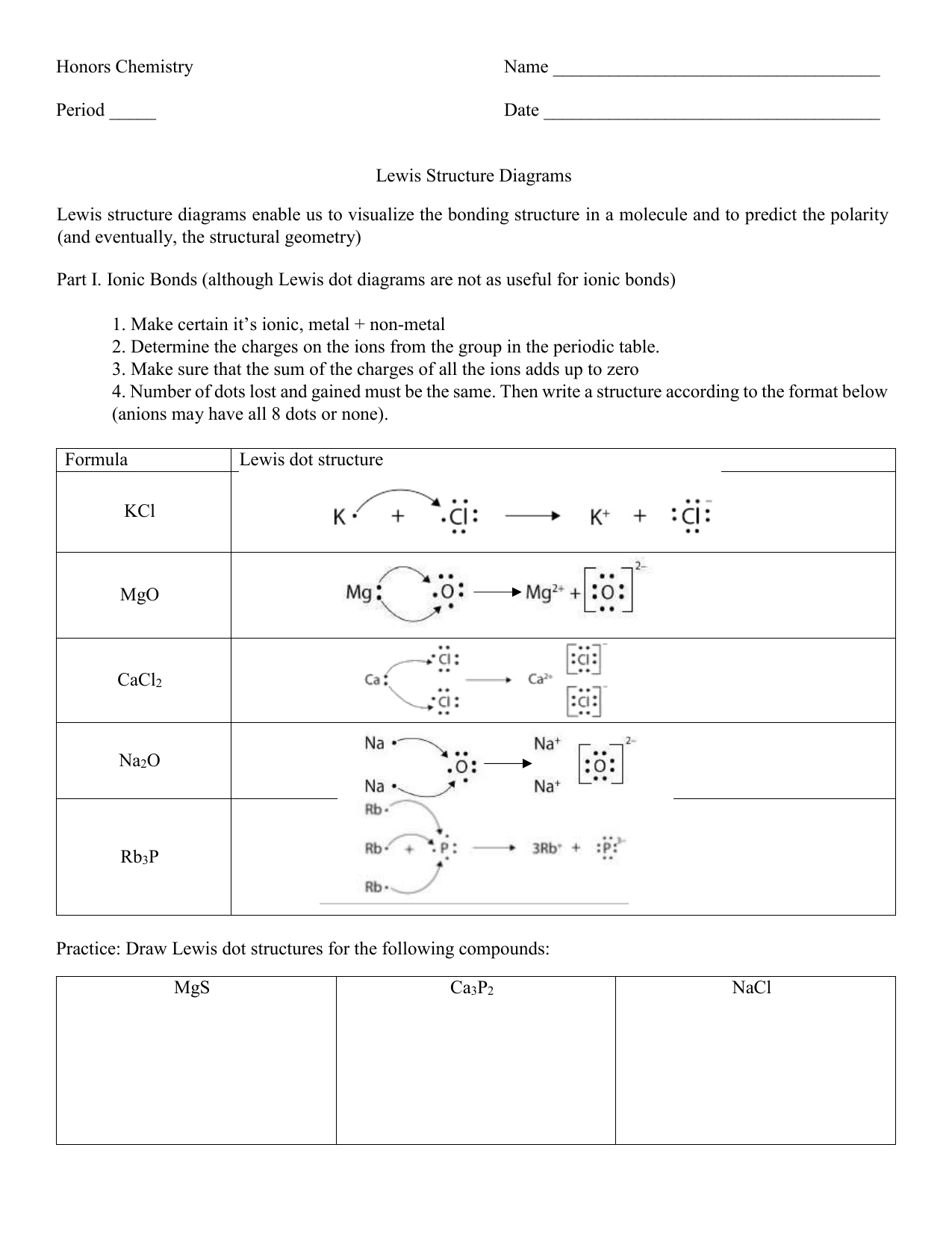
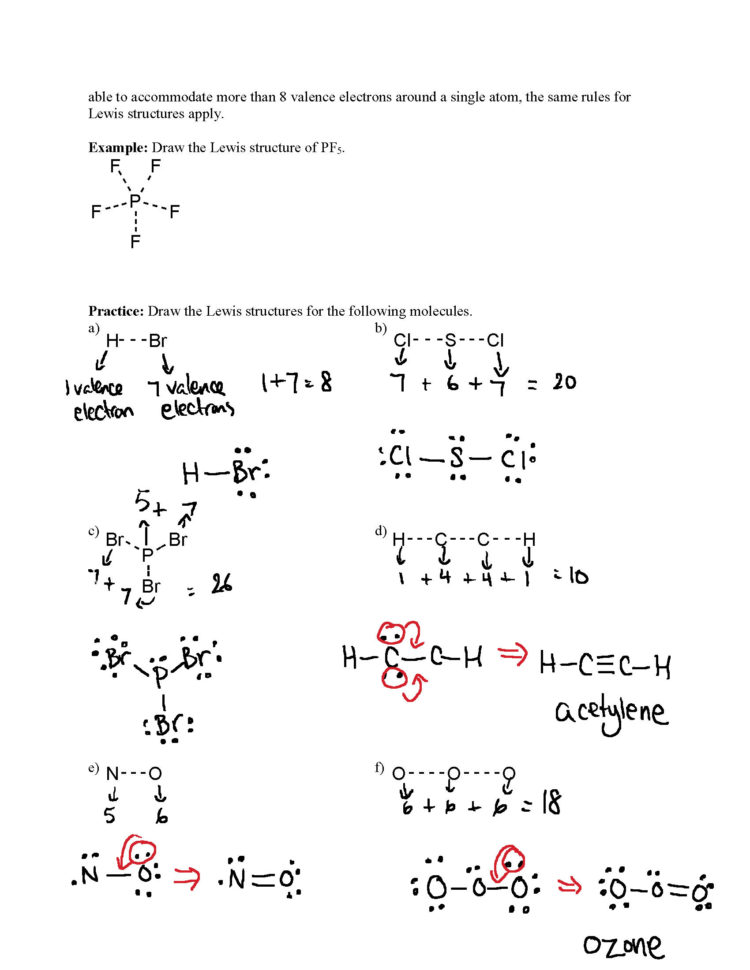
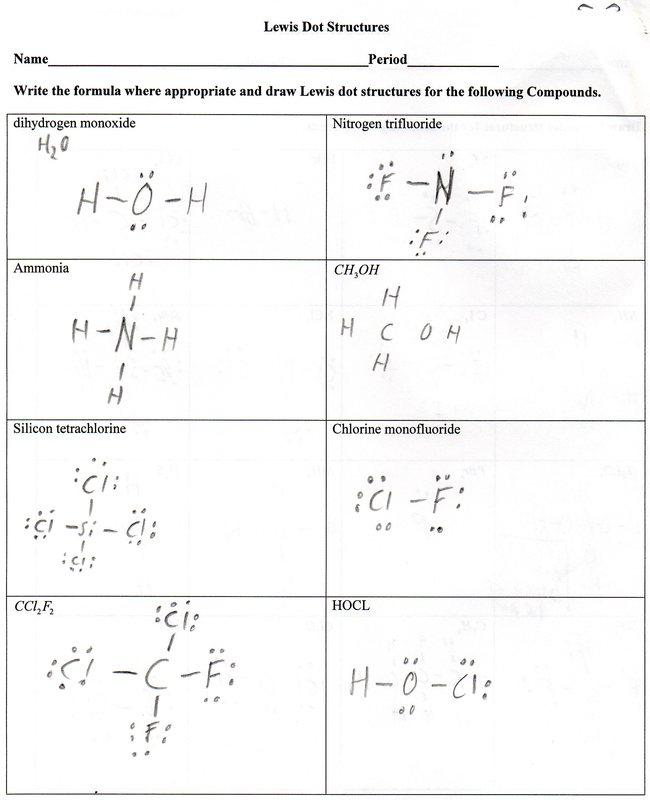
LEWIS STRUCTURES PRACTICE WORKSHEET Draw the Lewis Structures for each of the following molecules. If you are not sure if your structure is correct, do a formal charge check. You should consult the Lewis structure rules and a periodic table while doing this exercise.

Lewis Structure Practice Worksheet Answers Wiring Diagram Schemas
Practice Problems 2. Draw the Lewis dot structures for each of the following molecules: a. H2S c. SO3 b. CH2Br2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH4 + c. PO4 -3 b. NO3 - d. CO3 2- 4. For the following molecules or ions (where the central atom is underlined): Draw the Electron dot structure.
[Solved] Lewis Structure Practice Directions Complete the Lewis Structure... Course Hero
Lewis Dot Structure Practice Problems (with answers and explanation) Wayne Breslyn 724K subscribers Subscribe Subscribed 5.4K 484K views 5 years ago Practice drawing Lewis Structures.

Lewis Structure Practice Worksheet 50 Lewis Structure Practice Worksheet In 2020 Chemistry
1. Write the correct skeletal structure for the molecule. * Hydrogen atoms are always terminal (only one bond) * Put more electronegative elements in terminal positions 2. Sum the valence electrons from all the atoms. 3. Use a pair of electrons to form a bond between each pair of bound atoms. 4.

How To Draw 3D Lewis Structures Draw Lewis Structure C3h6 / Lewis, who introduced it in his
In short, these are the steps you need to follow for drawing a Lewis structure: 1. Write the correct skeletal structure for the molecule. * Hydrogen atoms are always terminal (only one bond) * Put more electronegative elements in terminal positions. 2. Sum the valence electrons from all the atoms. 3.
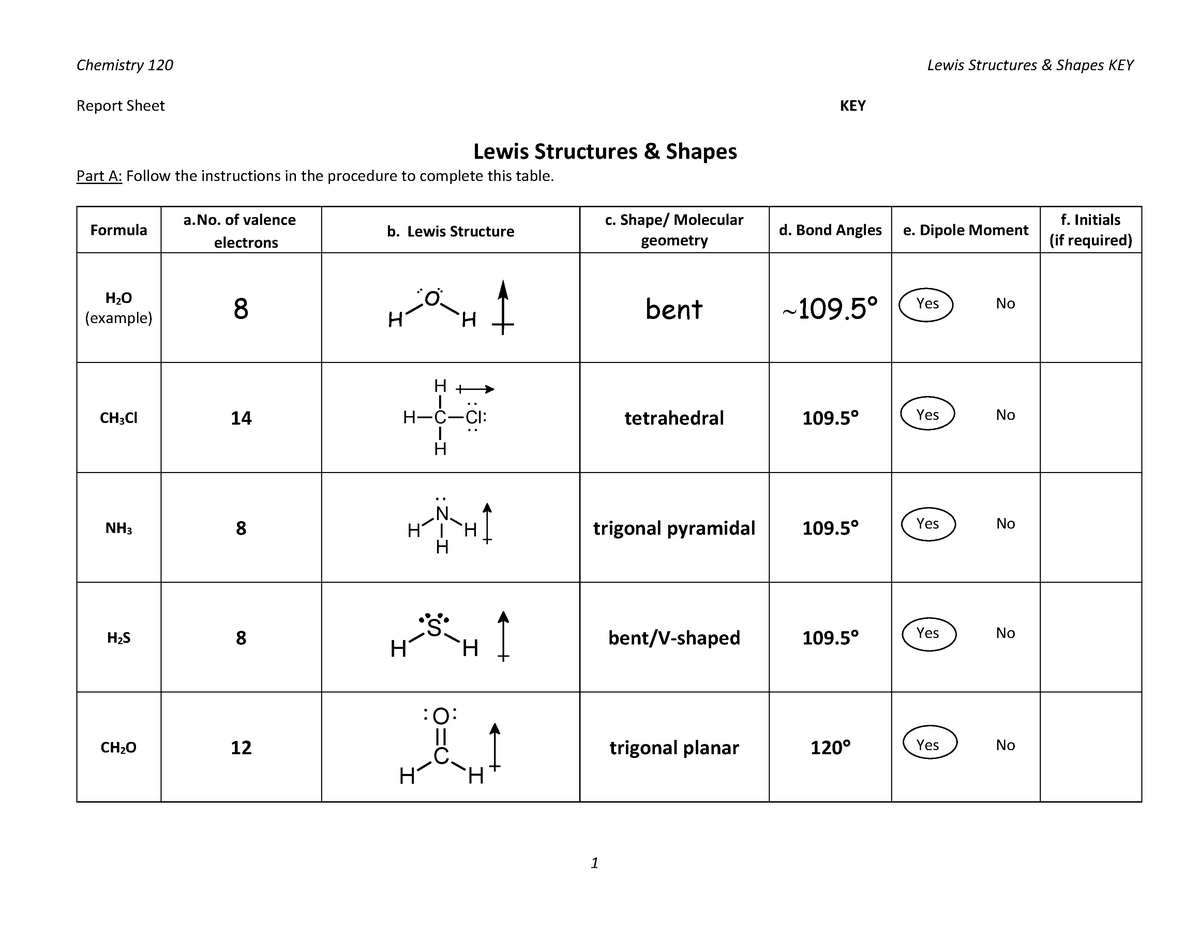
Lewis Structures Key Report Sheet KEY Lewis Structures & Shapes Part A Follow the Studocu
1.2.3 Guidelines about Formal Charges in Lewis Structures. The purpose of formal charges is to compare the difference between the number of valence electrons in the free atom and the number of electrons the atom "owns" when it is bonded. The smaller the difference, the "happier" (more stable) the atom is. The atom owns all of the lone pair (non-bonding) electrons and half of the.
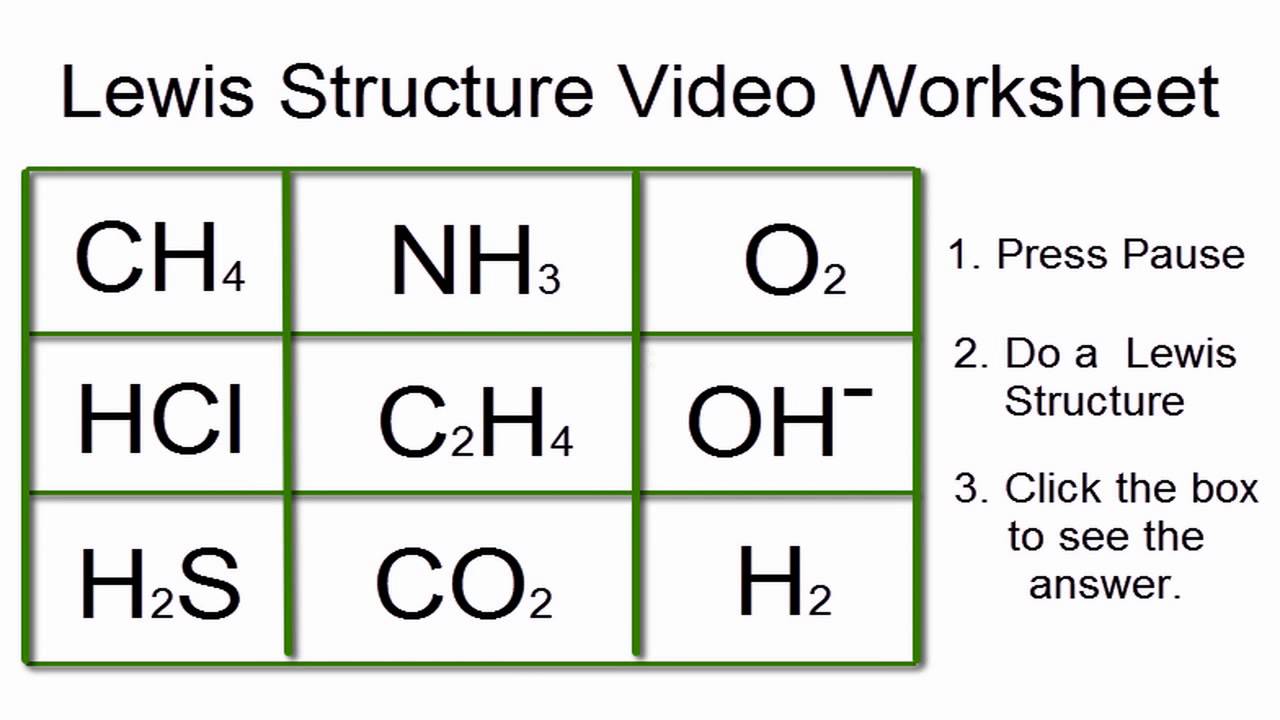
worksheet more lewis structures answers
This organic chemistry video tutorial explains how to draw lewis structures using a simple method. Organic Chemistry - Basic Introduction: https:.
covalent bond practice worksheet answers
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

Chemistry Lewis Structure Worksheet
A Compounds 1 and 2 are both polar. Compounds 1 and 2 are both nonpolar. B Compounds 1 and 2 are both nonpolar. Compound 1 is polar, whereas compound 2 is nonpolar. C Compound 1 is polar, whereas compound 2 is nonpolar. Compound 1 is nonpolar, whereas compound 2 is polar. D Compound 1 is nonpolar, whereas compound 2 is polar. Show Periodic Table

LEWIS STRUCTURES PRACTICE WORKSHEETthe Lewis structure rules and a periodic table while doing
Lewis diagrams. Google Classroom. You might need: Periodic table. Ethanethiol, C A 2 H A 6 S , is a clear liquid with a strong odor. The compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks. The skeletal structure of ethanethiol is shown below.

Lewis Structure Practice Worksheet Lewis Dot Diagrams Chemistry Handout Answers Diagram Base
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Chemistry Lewis Structure Worksheet
Practise drawing the Lewis structure of molecules using the exercises below. Select answers to see the correct drawings.